Preimplantation Genetic Testing (PGT), in conjunction with In Vitro Fertilization (IVF), aims to increase the likelihood of a successful pregnancy by identifying and transferring genetically normal embryos while decreasing the chances of transferring genetically abnormal embryos.
PGT has been proposed for various patient groups at risk of having genetically abnormal embryos, such as women of advanced maternal age (35 or older), those with a history of recurrent pregnancy loss (three or more), repeated failed IVF cycles with high-quality embryos (three or more transfers), and severe male factor infertility.
However, the use of PGT to test for genetic abnormalities in embryos has not consistently improved clinical outcomes and may even have some drawbacks. It has been reported that up to 16% of the results obtained through genetic analysis may be false positives, leading to the inadvertent discarding of embryos that could have resulted in a live birth.
One possible explanation for this phenomenon is embryo mosaicism, where an embryo contains both normal and abnormal cells. Sometimes, abnormal cell lines fail to proliferate, and the embryo can still develop into a normal offspring. If the biopsy for genetic testing is taken from an abnormal cell line, the embryo might be discarded, although it could have resulted in a healthy birth.
The application of PGT in specific cases, such as advanced maternal age, recurrent pregnancy loss, and repeated implantation failure, has not demonstrated clear benefits.
PGT has not shown a significant improvement in live birth rates for patients of advanced maternal age compared to the control group. Similarly, there is insufficient evidence for recurrent pregnancy loss and repeated implantation failure to support the use of PGT to improve outcomes.
PGT for male factor infertility has not been thoroughly studied, and there is not enough evidence to recommend its use for couples undergoing IVF with intracytoplasmic sperm injection (ICSI) due to male-factor indications.
PGT remains a personal choice for couples, particularly those without a history of repeated pregnancy losses. For these couples, PGT can be used to reduce the probability of miscarriage in IVF pregnancies.
PGT Procedure
Preimplantation Genetic Testing is a complex treatment consisting of the following steps:
1. In Vitro Fertilization to create embryos
2. Extended embryo culture to blastocyst
The embryos should reach the blastocyst stage (80 or more cells) by the fifth to seventh day after the egg retrieval.
The picture below shows an advanced stage of blastocyst development. Notice the central fluid-filled cavity. The cells within the blastocyst have already differentiated into the inner cell mass (at seven o’clock) that will give rise to the fetus and the trophectoderm cells that will form the future placenta.

3. Blastocyst trophectoderm (embryonic tissue) biopsy
An embryo biopsy is performed by creating an opening in the eggshell around the embryo. It is possible to safely remove six to eight cells through this opening using a special microscope with micromanipulators. The cells are taken from the trophectoderm only (future placenta cells, genetically identical to embryonic cells). The “inner mass” cells (embryonic cells) are not removed.
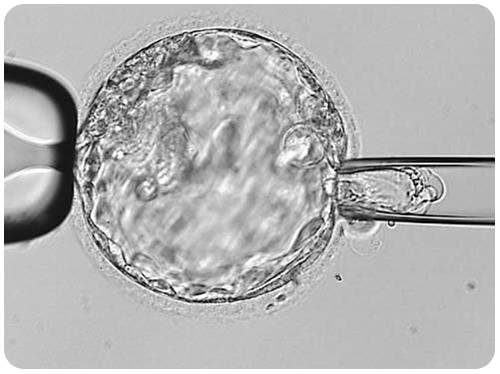
So far, there is no evidence that embryonic biopsy results in an increased chance of abnormalities in the baby or that the risk of birth defects is higher when compared to conceptions that occur spontaneously without medical assistance (2% to 5%).
4. Blastocyst vitrification (cryopreservation)
Since it takes several days to carry out the embryo genetic analysis, the blastocysts are cryopreserved immediately after their biopsy and stored in liquid nitrogen.
5. Genetic analysis of the embryonic tissue
The accuracy of PGT cell analysis of genetically abnormal embryos approaches 100% but is not guaranteed. Even though highly unlikely, it is possible that an embryo tested as normal may not be genetically perfect.
6. Liquid nitrogen storage of the cryopreserved embryos
Vitrified embryos can be stored for extended periods, but most PGT patients will start an embryo transfer cycle with the onset of the first menstrual period after IVF treatment.
7. Subsequent Frozen Embryo Transfer
The endometrial lining is first stimulated with estrogen and progesterone, followed by the thawing of one or two embryos tested as normal by the PGT analysis and then transferring them inside the uterus.
Ultimately, PGT represents a valuable tool for identifying genetic abnormalities in embryos and can significantly benefit certain patient groups. However, it is essential to carefully consider its application and potential limitations, especially in cases where the benefits have not been clearly demonstrated.